





The idea of pooling certain heavy equipment in the context of an experimental platform located on the site of the Faculty of Sciences and Technologies in Nancy emerged shortly after the creation of the University of Lorraine (UL) in 2012 as part of the State/Regional Plan Contract (CPER) FORBOIS and the European Regional Development Fund (ERDF).
From 2017 onwards, the construction of this platform was given a new impetus. Four research units (UMR 1136 IAM, UMR 1128 DynAMic, UMR 1434 SILVA and USC INRAE 340 L2A) have pooled human and financial resources to structure the platform.
ASIA “Functional and Structural Approaches to Cellular InterActions” is a scientific platform whose objective is to provide access to cutting-edge technological resources and to bring high-level expertise to users. The platform welcomes students from a wide range of courses at the Université de Lorraine.
It is equipped with facilities for the study of the regulation of biological and physiological activities of proteins as well as the study of cellular interactions. The tools available on the platform make it possible to address these questions at different levels.
Attached to the A2F scientific cluster, the ASIA platform is accessible to all users whatever their affiliation (public bodies, companies, etc.). A quality approach has been put in place in order to satisfy users as much as possible.
Find the tool you need!
Equipment
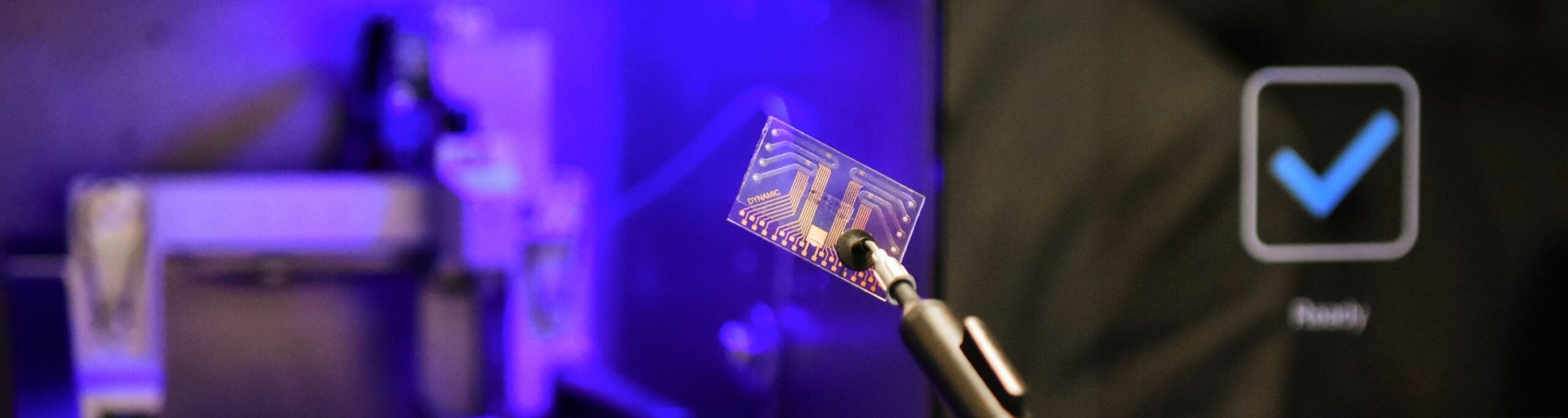
switchSENSE® heliX+
switchSENSE® technology utilizes a electro-switchable biosurface to provide researchers and commercial laboratories the ability to characterize interactions between molecules in real-time. This technology is unlike existing methodologies in that it combines high sensitivity kinetics with structural information on size, shape and conformation providing a new depth and understanding of the interaction. Studies are performed on a re-usable biochip, generated using familiar coupling and hybridization methods. Within this biochip, DNA levers are embedded onto a series of gold electrodes. These nanolevers serve either as target for molecular interactions themselves, or hold other interaction partners. To characterize interactions, the DRX instrument is used to bring about deliberate movement of these nanolevers by altering the voltage across the gold surface. When interactions occur, these movements are affected and in turn, used in the calculation of kinetic and biophysical information.
For further information, please visit www.dynamic-biosensors.com
switchSENSE® DRX
switchSENSE® technology utilizes a novel electro-switchable biosurface to provide researchers and commercial laboratories the ability to characterize interactions between molecules in real-time. This technology is unlike existing methodologies in that it combines high sensitivity kinetics with structural information on size, shape and conformation providing a new depth and understanding of the interaction. Studies are performed on a re-usable biochip, generated using familiar coupling and hybridization methods. Within this biochip, DNA levers are embedded onto a series of gold electrodes. These nanolevers serve either as target for molecular interactions themselves, or hold other interaction partners. To characterize interactions, the DRX instrument is used to bring about deliberate movement of these nanolevers by altering the voltage across the gold surface. When interactions occur, these movements are affected and in turn, used in the calculation of kinetic and biophysical information.
For further information, please visit www.dynamic-biosensors.com
ITC
The isothermal titration calorimetry technology or ITC (MicroCal ITC200 apparatus) is used in quantitative studies of a wide variety of biomolecular interactions (assemblies of macromolecules such as proteins or nucleic acids, protein/small molecule interactions, etc.). It allows to measure the affinity of binding partners in their native state. It
is based on a direct measurement of the heat released or absorbed during a biomolecule binding event. The measurement of heat transfer during binding allows an accurate determination of the affinity constant (KA = 1/KD), stoichiometry (n), enthalpy (∆H) and entropy (-TΔS) of the reaction and thus the Gibbs free energy of the biological system (∆G0). This provides a complete thermodynamic profile of the parameters of the molecular interaction. This technology is complementary to the switchSENSE® technology also available on the platform.
Analytical uhplc-ms

The enzymatic activity of proteins can be analyzed using High Performance Liquid Chromatography or HPLC (Acquity Arc Apparatus) technology coupled with detection of
mass spectrophotometry (Acquity QDa apparatus) by measuring and identifying certain products formed during the reaction. The UHPLC technology (for ultra-high performance liquid chromatography) works with columns filled with silica particles of sub-2 µm diameter and at high pressures of 600-650 bar. It thus offers a much higher resolution than conventional HPLC (100-150 bar). UHPLC is therefore a technology perfectly suited to the fractionation of complex mixtures. It allows the separation and dosage of numerous metabolites resulting from primary and secondary metabolism (amino acids, carbohydrates, aromatic compounds, organic acids) or involved in cellular detoxification mechanisms (antioxidants such as ascorbate, glutathione, etc.). Proteins and their degradation products (peptides) can also be separated and dosed. However, Acquity-Arc does not allow the collection of fractions at the exit of the column.
Preparative uhplc-ms

The enzymatic activity of proteins can be analyzed using High Performance Liquid Chromatography or HPLC (Acquity Arc Apparatus) technology coupled with detection of
mass spectrophotometry (Acquity QDa apparatus) by measuring and identifying certain products formed during the reaction. The UHPLC technology (for ultra-high performance liquid chromatography) works with columns filled with silica particles of sub-2 µm diameter and at high pressures of 600-650 bar. It thus offers a much higher resolution than conventional HPLC (100-150 bar). UHPLC is therefore a technology perfectly suited to the fractionation of complex mixtures. It allows the separation and dosage of numerous metabolites resulting from primary and secondary metabolism (amino acids, carbohydrates, aromatic compounds, organic acids) or involved in cellular detoxification mechanisms (antioxidants such as ascorbate, glutathione, etc.). Proteins and their degradation products (peptides) can also be separated and dosed.
Flash chromatography

Flash chromatography (PuriFlash XS420 apparatus) is a technique used to fractionate a crude sample (culture medium, plant extracts, food matrices in solution, etc.) and
obtain fractions highly enriched in a compound with a given biological activity. It is a simple and fast separation technique. Flash chromatography is based on the interaction between the compounds to be separated, the stationary phase (grafted or ungrafted silica beads) and the mobile phase (aqueous or organic solvent). The pressure required is low (less than 20 bar) compared to HPLC where it is higher than 100 bar.
SEC-MALS

Reference : Tiphaine DHALLEINE
Multi-angle light scattering or MALS (miniDAWN TREOS II detector) coupled to an FPLC system (ÄKTA-purifier apparatus) is a non-destructive spectroscopic analysis technique for measuring the molecular weight of eluted molecules (proteins and possibly nucleic acids), to monitor the quality of a purification, to measure the stoichiometry of macromolecular complexes, to measure the state of oligomerisation, to monitor the homogeneity of the size of the eluted molecule, to monitor possible changes in conformation in the presence of effectors. It also makes it possible to highlight possible conformational changes in the molecule under study (a powerful analytical tool for complexes and modular proteins) and finally to obtain structural data on the interactions between molecules in solution.
FPLC ÄKTA pure

The Fast Protein Liquid Chromatography or FPLC technology (ÄKTA-purifier and ÄKTA-pure 25L devices) enables the separation of macromolecules such as proteins (natural or recombinant) and nucleic acids. Analytical or preparative columns can be used. This technology preserves the native character of the proteins and their potential biological activity as it is specially designed in inert materials (glass, Teflon, PEEK tubes) and works with medium back pressures (1 MPa or 10 bar). The most commonly used techniques are anion or cation exchange, hydrophobic interaction, gel filtration (or SEC steric exclusion), immobilized nickel ion affinity chromatography for His-tagged proteins (IMAC).
Bioinformatics station

Référent : Sylvain Darnet
Station de travail tour
Precision 3660
Base : Precision 3660, base BTX
Processeur : Processeur Intel Core I9-13900K de 13e génération (cache 36 Mo, 24 cœurs, 32 threads, 3,00 GHz à 5,80 GHz Turbo, 125W)
Système d’exploitation : Windows 11 Professionnel, anglais, néerlandais, français, allemand, Italien,
Option de châssis : Precision 3660 Tour avec bloc d’alimentation 1000 W (80 Plus platinium), compatible RPL et ADL, V2
Carte vidéo : Carte graphique intel intégrée
Thermal Coolin : Refroidisseur d’air thermique de processeur avancé
Mémoire : 32 Go (2 x 16 Go) de mémoire DDR5 UDIMM non ECC jusqu’à 4400 MHz
Storage Configuration (Boot Drive) : C5 : disque SSD M.2 en option + disque du SATA 3,5°
1st M.2 NVMe SSD : Disque SSD M.2 PCle NVMe classe 40 de 1 To
Additional M.2 NVMe SSD : Double disque SSD M.2 PCle NVMe classe 40 de 1 To
Disque dur : pas de disque dur
Disque dur supplémentaire : Disque dur SATA 3,5 pouces de 4 To à 5400 tr/min
3rd Hard Disk Drive : Pas de disque dur
Connectivité RAID : Sans RAID SATA
Lecteur optique : Lecteur de DVD+/-RW 8x 9,5 mm
Logiciel optique : Logiciel Cyberlink Media Suite Essentials pour Windows 10 et lecteur DVD (sans support)
Additional Network Add-in-cards : Aucune carte reseau supplémentaire sélectionnée (carte d’interface réseau intégrée incluse)
Sans fil : Aucune carte LAN sans fil incluse (aucune connectivité WI-FI)
Pilote pour technologie sans fil : Pas de carte réseau LAN sans fils
PCle I/O Add-in-cards : Non sélectionné dans cette configuration
Optional Integrated Video or USB Ports : DisplayPort supplémentaire
Haut-parleurs : haut-parleur intégré pour Precision 3660
Capot arrière : Aucune gaine de câble sécurisée incluse
Ecran incurvé 49 pouces, Haute résolution
Logiciel : XL Stat
Nephelometer

For high-throughput (microplate) screening of microbial growth, molecular solubility and protein binding kinetics.
Fluorescence spectrometer

A fluorescence spectrometer measures the fluorescence re-emitted at a given wavelength.
Microplate reader

The microplate reader (6 to 384 wells) allows absorbance measurements between 230-800 nm and fluorescence measurements between 203-800 nm. It allows absorption spectra and fluorescence excitation and emission spectra to be performed.
ultracentrifuge

Separation of macromolecules (nucleic acids, lipoproteins, etc.) and cellular organelles by centrifugal acceleration (5000 g to 100,000 g).
Automatic TLC Sampler

The ATS 4 Laboratory Autosampler from Camag is a device for fully automated application of samples to TLC and HPTLC plates. The samples can be applied as strips, dots or rectangles. Automatic sample application is a key factor for the productivity of the HPTLC laboratory. The ATS 4 offers fully automatic sample application for qualitative and quantitative analyses as well as preparative separations. It is suitable for routine use and high sample throughput. Key features Fully automated sample application Strip, dot or rectangle application Any plate size up to 20 x 20 cm Spray or contact transfer application Controlled by visionCATS software Heated spray nozzle (optional) ATS 4 operation with visionCATS Accurate sample application is a crucial factor for the quality of HPTLC analysis. By using the visionCATS HPTLC software to control the ATS 4, a fully automated sample application for routine use and high sample throughput is supported. The dialog box for the instrument parameters offers easy-to-use default combinations. For example, by selecting the solvent type most similar to the solvent actually used, the software automatically adapts the instrument default settings based on viscosity, volatility and surface tension. The filling/rinsing quality, which determines how often the syringe is rinsed, how often the filling process is repeated, etc., can be individually adjusted to a specific task. The sample table is clearly arranged and easy to use. The progress of the application is displayed on the screen as long as the instrument remains connected to the computer. Technical specifications Object holder For objects up to 20 x 20 cm.
Automatic derivatization machine

Chromajet DS20 Bionis derivatization machine.
Spraying ensures the control of the development step.
The ChromaJet DS 20 allows to spray derivatization reagents on very precise areas defined by the user. This technology, unique on the market, ensures substantial savings in reagent consumption for the user.
Thanks to the closed system of the Chromajet DS20, the user works in complete safety.
Vacuum concentrator

Concentration by vacuum centrifugation (miVAC equipment) allows rapid removal of organic solvent from samples.
Freeze dryer

Freeze-drying is the removal of water from a liquid, pasty or solid product by sublimation.
Cryogenic mill

Thanks to the integrated cooling system (liquid nitrogen at -196°C), the grinding bowl is continuously cooled with liquid nitrogen before and during grinding. The sample (food, wood, sludge, plant parts, oil seeds, soil, textiles, etc.) is thus weakened and volatile components are preserved.
Soxtherm
Solvent extraction of small molecules (polycyclic aromatic hydrocarbons, metabolites, carbohydrate polymers, terpenes, polyphenols, alkaloids…) is a classic solid-liquid extraction method. The sample quickly comes into contact with pure organic solvent, which helps to shift the transfer equilibrium to the solvent. It does not require filtration after extraction and can be used whatever the plant matrix.
The ASIA platform also has three Soxhlets (non-automated extraction devices).
qPCR : CFX 96 and 384
Development of primers; detection of point mutations; determination of GMOs in products for human consumption; specific and sensitive detection of pathogens and quantification of bacterial, viral or parasitic load.
Publications involving the platform
When promoting your scientific projects (articles, posters, presentations), don’t forget to thank the ASIA platform,
whether or not staff are co-authors. In order to facilitate the control of publications involving the platform, please use the following wording:
“This work benefited from the ASIA platform (University of Lorraine-INRAE; https://a2f.univ-lorraine.fr/en/asia-2/). »
Don’t forget to send a PDF copy of your scientific production to the operational manager. Thank you!
For recent publications the link will be updated soon
Cliquez sur le logo pour accéder aux publications :
![]() Caubrière, D., de Butler, A., Moseler, A., Leverrier, P., Collet, J. F., Meyer, A. J., Rouhier, N., & Couturier, J. (2025). Fusion of a bacterial cysteine desulfurase to redox-sensitive green fluorescent protein produces a highly sensitive cysteine biosensor for monitoring changes in intracellular cysteine. Redox biology, 85, 103785. https://doi.org/10.1016/j.redox.2025.103785
Caubrière, D., de Butler, A., Moseler, A., Leverrier, P., Collet, J. F., Meyer, A. J., Rouhier, N., & Couturier, J. (2025). Fusion of a bacterial cysteine desulfurase to redox-sensitive green fluorescent protein produces a highly sensitive cysteine biosensor for monitoring changes in intracellular cysteine. Redox biology, 85, 103785. https://doi.org/10.1016/j.redox.2025.103785
![]() Vandekerkhove, C., Bchini, R., Dhalleine, T., Kohler, A., Deveau, A., Pandharikar, G., Besserer, A., Sormani, R., Darnet, S., & Morel-Rouhier, M. (2025). Dissecting the mechanisms of copper-azole wood preservatives detoxification by ligninolytic fungi. Journal of hazardous materials, 486, 136934. https://doi.org/10.1016/j.jhazmat.2024.136934
Vandekerkhove, C., Bchini, R., Dhalleine, T., Kohler, A., Deveau, A., Pandharikar, G., Besserer, A., Sormani, R., Darnet, S., & Morel-Rouhier, M. (2025). Dissecting the mechanisms of copper-azole wood preservatives detoxification by ligninolytic fungi. Journal of hazardous materials, 486, 136934. https://doi.org/10.1016/j.jhazmat.2024.136934
![]() Camaño Echavarría J.A., Irankunda R., Arnoux P., Mathé C., Girardet J.M., Cakir-Kiefer C., Risler A., Stefan L., Paris C., Zapata Montoya J.E., Selmeczi K., & Canabady-Rochelle L. (2025). Characterization and Bioactivities of Gelatin Hydrolysates of Red Tilapia (Oreochromis spp.) Scale By-products. ACS Food Science & Technology, 5, 1100-1115. https://doi.org/10.1021/acsfoodscitech.4c00918
Camaño Echavarría J.A., Irankunda R., Arnoux P., Mathé C., Girardet J.M., Cakir-Kiefer C., Risler A., Stefan L., Paris C., Zapata Montoya J.E., Selmeczi K., & Canabady-Rochelle L. (2025). Characterization and Bioactivities of Gelatin Hydrolysates of Red Tilapia (Oreochromis spp.) Scale By-products. ACS Food Science & Technology, 5, 1100-1115. https://doi.org/10.1021/acsfoodscitech.4c00918
![]() Camaño Echavarría J.A., Mathé C., Girardet J.M., Paris C, Udenigwe C.C., Selmeczi K, & Canabady-Rochelle L. (2025). Identification of Ni2+-binding peptides in sunflower meal protein hydrolysate for deeper understanding of peptide-metal interactions. Journal of Inorganic Biochemistry 269, 112877. https://doi.org/10.1016/j.jinorgbio.2025.112877
Camaño Echavarría J.A., Mathé C., Girardet J.M., Paris C, Udenigwe C.C., Selmeczi K, & Canabady-Rochelle L. (2025). Identification of Ni2+-binding peptides in sunflower meal protein hydrolysate for deeper understanding of peptide-metal interactions. Journal of Inorganic Biochemistry 269, 112877. https://doi.org/10.1016/j.jinorgbio.2025.112877
![]() Irankunda R., Camaño Echavarría J.A., El Hajj S., Paris C., Cakir-Kiefer C., Girardet J.M., Arnoux P., Muhr L., Canabady-Rochelle L. (2025). Pisum Sativum Protein Hydrolysates: Production, Sensitive Screening of Nickel Chelating Peptides and Evaluation of Antioxidant Properties. Food Bioscience, 106001. https://doi.org/10.1016/j.fbio.2025.106001.
Irankunda R., Camaño Echavarría J.A., El Hajj S., Paris C., Cakir-Kiefer C., Girardet J.M., Arnoux P., Muhr L., Canabady-Rochelle L. (2025). Pisum Sativum Protein Hydrolysates: Production, Sensitive Screening of Nickel Chelating Peptides and Evaluation of Antioxidant Properties. Food Bioscience, 106001. https://doi.org/10.1016/j.fbio.2025.106001.
![]() Maffo-Woulefack R, Ali AM, Laroussi H, et al. Elucidating assembly and function of VirB8 cell wall subunits refines the DNA translocation model in Gram-positive T4SSs. Sci Adv. 2025;11(4):eadq5975. doi:10.1126/sciadv.adq5975
Maffo-Woulefack R, Ali AM, Laroussi H, et al. Elucidating assembly and function of VirB8 cell wall subunits refines the DNA translocation model in Gram-positive T4SSs. Sci Adv. 2025;11(4):eadq5975. doi:10.1126/sciadv.adq5975
![]() Joffe, R., Tosens, T., Berthe, A., Jolivet, Y., Niinemets, Ü., & Gandin, A. (2024). Reduced mesophyll conductance under chronic O3 exposure in poplar reflects thicker cell walls and increased subcellular diffusion pathway lengths according to the anatomical model. Plant, Cell & Environment, 47(12), 4815‑4832. https://doi.org/10.1111/pce.15049
Joffe, R., Tosens, T., Berthe, A., Jolivet, Y., Niinemets, Ü., & Gandin, A. (2024). Reduced mesophyll conductance under chronic O3 exposure in poplar reflects thicker cell walls and increased subcellular diffusion pathway lengths according to the anatomical model. Plant, Cell & Environment, 47(12), 4815‑4832. https://doi.org/10.1111/pce.15049
Baratzhanova, G., Girardet, J.-M., Fournier, A., Djansugurova, L., & Cakir-Kiefer, C. (2024). Application of the switchSENSE® technology for real-time study of pesticides interaction with biological molecules. BIO Web of Conferences, 100, 03003. https://doi.org/10.1051/bioconf/202410003003
Bjørlie, M., Irankunda, R., Yesiltas, B., Sørensen, A.-D. M., Girardet, J.-M., Boschi-Müller, S., Jacobsen, C., & Canabady-Rochelle, L. (2024). Metal-chelating antioxidant peptides—Biosensor screening methods as alternatives to the ferrozine assay. https://doi.org/10.22541/au.171223997.71294832/v1
Bc![]() hini, R., Darnet, S., De Butler, A., Doan, A., Oliveira-Correia, L., Navarro, D., Record, E., & Morel-Rouhier, M. (2024). Responses to and detoxification of esculin in white-rot fungi. Fungal Biology, S1878614623001393. https://doi.org/10.1016/j.funbio.2023.12.008
hini, R., Darnet, S., De Butler, A., Doan, A., Oliveira-Correia, L., Navarro, D., Record, E., & Morel-Rouhier, M. (2024). Responses to and detoxification of esculin in white-rot fungi. Fungal Biology, S1878614623001393. https://doi.org/10.1016/j.funbio.2023.12.008
Cliquez sur le logo pour accéder aux publications
![]() Safran J., Tabi W., Ung V., Lemaire A., Habrylo O., Bouckaert J., Rouffle M., Voxeur A., Pongrac P., Bassard S., Molinié R., Fontaine J.-X., Pilard S., Pau-Roblot C., Bonnin E., Larsen D.S., Morel-Rouhier M., Girardet J.M., Lefebvre V., Sénéchal F., Mercadante D., & Pelloux J. (2023). Differences in the crystal structure of plant polygalacturonases specify enzymes’ dynamics and processivities to fine-tune cell wall pectins. The Plant Cell. https://doi.org/10.1101/2022.06.22.497136
Safran J., Tabi W., Ung V., Lemaire A., Habrylo O., Bouckaert J., Rouffle M., Voxeur A., Pongrac P., Bassard S., Molinié R., Fontaine J.-X., Pilard S., Pau-Roblot C., Bonnin E., Larsen D.S., Morel-Rouhier M., Girardet J.M., Lefebvre V., Sénéchal F., Mercadante D., & Pelloux J. (2023). Differences in the crystal structure of plant polygalacturonases specify enzymes’ dynamics and processivities to fine-tune cell wall pectins. The Plant Cell. https://doi.org/10.1101/2022.06.22.497136
![]() Schilling, M., Maia-Grondard, A., Baltenweck, R., Robert, E., Hugueney, P., Bertsch, C., Farine, S., & Gelhaye, E. (2022). Wood degradation by Fomitiporia mediterranea M. Fischer: Physiologic, metabolomic and proteomic approaches. Frontiers in plant science, 13, 988709. https://doi.org/10.3389/fpls.2022.988709
Schilling, M., Maia-Grondard, A., Baltenweck, R., Robert, E., Hugueney, P., Bertsch, C., Farine, S., & Gelhaye, E. (2022). Wood degradation by Fomitiporia mediterranea M. Fischer: Physiologic, metabolomic and proteomic approaches. Frontiers in plant science, 13, 988709. https://doi.org/10.3389/fpls.2022.988709
![]() Sylvestre-Gonon, E., Morette, L., Viloria, M., Mathiot, S., Boutilliat, A., Favier, F., Rouhier, N., Didierjean, C., & Hecker, A. (2022). Biochemical and Structural Insights on the Poplar Tau Glutathione Transferase GSTU19 and 20 Paralogs Binding Flavonoids. Frontiers in molecular biosciences, 9, 958586. https://doi.org/10.3389/fmolb.2022.958586
Sylvestre-Gonon, E., Morette, L., Viloria, M., Mathiot, S., Boutilliat, A., Favier, F., Rouhier, N., Didierjean, C., & Hecker, A. (2022). Biochemical and Structural Insights on the Poplar Tau Glutathione Transferase GSTU19 and 20 Paralogs Binding Flavonoids. Frontiers in molecular biosciences, 9, 958586. https://doi.org/10.3389/fmolb.2022.958586
![]() Narmuratova Z., Hentati F., Girardet J.M., Narmuratova M., & Cakir-Kiefer C. (2022). Equine lactoferrin: Antioxidant properties related to divalent metal chelation. LWT Food Science and Technology, 161, 113426.
Narmuratova Z., Hentati F., Girardet J.M., Narmuratova M., & Cakir-Kiefer C. (2022). Equine lactoferrin: Antioxidant properties related to divalent metal chelation. LWT Food Science and Technology, 161, 113426.
![]() Joffe, R., Berthe, A., Jolivet, Y., & Gandin, A. (2022). The response of mesophyll conductance to ozone-induced oxidative stress is genotype-dependent in poplar. Journal of experimental botany, erac154. Advance online publication. https://doi.org/10.1093/jxb/erac154
Joffe, R., Berthe, A., Jolivet, Y., & Gandin, A. (2022). The response of mesophyll conductance to ozone-induced oxidative stress is genotype-dependent in poplar. Journal of experimental botany, erac154. Advance online publication. https://doi.org/10.1093/jxb/erac154
![]() Su, L., Hôtel, L., Paris, C., Chepkirui, C., Brachmann, A. O., Piel, J., Jacob, C., Aigle, B., & Weissman, K. J. (2022). Engineering the stambomycin modular polyketide synthase yields 37-membered mini-stambomycins. Nature communications, 13(1), 515. https://doi.org/10.1038/s41467-022-27955-z
Su, L., Hôtel, L., Paris, C., Chepkirui, C., Brachmann, A. O., Piel, J., Jacob, C., Aigle, B., & Weissman, K. J. (2022). Engineering the stambomycin modular polyketide synthase yields 37-membered mini-stambomycins. Nature communications, 13(1), 515. https://doi.org/10.1038/s41467-022-27955-z
![]() Pacetti, A., Moretti, S., Pinto, C., Compant, S., Farine, S., Bertsch, C., & Mugnai, L. (2021). Trunk Surgery as a Tool to Reduce Foliar Symptoms in Diseases of the Esca Complex and Its Influence on Vine Wood Microbiota. Journal of fungi (Basel, Switzerland), 7(7), 521. https://doi.org/10.3390/jof7070521
Pacetti, A., Moretti, S., Pinto, C., Compant, S., Farine, S., Bertsch, C., & Mugnai, L. (2021). Trunk Surgery as a Tool to Reduce Foliar Symptoms in Diseases of the Esca Complex and Its Influence on Vine Wood Microbiota. Journal of fungi (Basel, Switzerland), 7(7), 521. https://doi.org/10.3390/jof7070521
![]() El Hajj S., Sepúlveda-Rinconi T., Girardet J.M., Cakir-Kiefer C., Stefan L., Zapata-Montoya J.E., Boshi-Muller S., Gaucher C. & Canabady-Rochelle L. (2021) Electrically switchable nanolever technology for the screening of metal-chelating peptides in hydrolysates. Journal of Agricultural and Food Chemistry, 69, 8819-8827.
El Hajj S., Sepúlveda-Rinconi T., Girardet J.M., Cakir-Kiefer C., Stefan L., Zapata-Montoya J.E., Boshi-Muller S., Gaucher C. & Canabady-Rochelle L. (2021) Electrically switchable nanolever technology for the screening of metal-chelating peptides in hydrolysates. Journal of Agricultural and Food Chemistry, 69, 8819-8827.
![]() Bchini R., Girardet J.M., Sormani R., Gelhaye E. & Morel-Rouhier M. (2021). Oxidized glutathione promotes association between Eukaryotic Translation Elongation Factor 1Bγ and Ure2p glutathione transferase from Phanerochaete chrysosporium. The FEBS Journal, 288, 2956-2969.Bchini R., Girardet J.M., Sormani R., Gelhaye E. & Morel-Rouhier M. (2021). Oxidized glutathione promotes association between Eukaryotic Translation Elongation Factor 1Bγ and Ure2p glutathione transferase from Phanerochaete chrysosporium. The FEBS Journal, 288, 2956-2969.
Bchini R., Girardet J.M., Sormani R., Gelhaye E. & Morel-Rouhier M. (2021). Oxidized glutathione promotes association between Eukaryotic Translation Elongation Factor 1Bγ and Ure2p glutathione transferase from Phanerochaete chrysosporium. The FEBS Journal, 288, 2956-2969.Bchini R., Girardet J.M., Sormani R., Gelhaye E. & Morel-Rouhier M. (2021). Oxidized glutathione promotes association between Eukaryotic Translation Elongation Factor 1Bγ and Ure2p glutathione transferase from Phanerochaete chrysosporium. The FEBS Journal, 288, 2956-2969.
![]() El Hajj S., Sepúlveda-Rinconi T., Selmeczi K., Paris C., Giraud T., Csire G., Stefan L., Girardet J.M., Desobry S., Bouhallab S., Muhr L., Gaucher C. & Canabady-Rochelle L. (2021) Chapter 19, Applications in Nutrition: Mineral-Binding. In Biologically Active Peptides 1st Ed.: From basic science to applications for human health (Fidel Toldrá and Jianping Wu, Eds.), pp 455-494, Elsevier. Academic Press.
El Hajj S., Sepúlveda-Rinconi T., Selmeczi K., Paris C., Giraud T., Csire G., Stefan L., Girardet J.M., Desobry S., Bouhallab S., Muhr L., Gaucher C. & Canabady-Rochelle L. (2021) Chapter 19, Applications in Nutrition: Mineral-Binding. In Biologically Active Peptides 1st Ed.: From basic science to applications for human health (Fidel Toldrá and Jianping Wu, Eds.), pp 455-494, Elsevier. Academic Press.
![]() Cappele J., Mohamad Ali A., Leblond-Bourget N., Mathiot S., Dhalleine T., Payot S., Savko M., Didierjean C., Favier F., Douzi B. (2021). Structural and Biochemical Analysis of OrfG: The VirB8-like Component of the Conjugative Type IV Secretion System of ICESt3 From Streptococcus thermophilus. Frontiers in Molecular Biosciences, 80, 103389.
Cappele J., Mohamad Ali A., Leblond-Bourget N., Mathiot S., Dhalleine T., Payot S., Savko M., Didierjean C., Favier F., Douzi B. (2021). Structural and Biochemical Analysis of OrfG: The VirB8-like Component of the Conjugative Type IV Secretion System of ICESt3 From Streptococcus thermophilus. Frontiers in Molecular Biosciences, 80, 103389.
![]() Perrot T., Schwartz M., Deroy A., Girardet J.M., Kohler A., Morel-Rouhier M., Favier F., Gelhaye E. & Didierjean C. (2021). Diversity of Omega Glutathione Transferases in mushroom-forming fungi revealed by phylogenetic, transcriptomic, biochemical and structural approaches. Fungal Genetics and Biology, 148, 103506.
Perrot T., Schwartz M., Deroy A., Girardet J.M., Kohler A., Morel-Rouhier M., Favier F., Gelhaye E. & Didierjean C. (2021). Diversity of Omega Glutathione Transferases in mushroom-forming fungi revealed by phylogenetic, transcriptomic, biochemical and structural approaches. Fungal Genetics and Biology, 148, 103506.
![]() Risser, F., Collin, S., Dos Santos-Morais, R., Gruez, A., Chagot, B., & Weissman, K. J. (2020). Towards improved understanding of intersubunit interactions in modular polyketide biosynthesis: Docking in the enacyloxin IIa polyketide synthase. Journal of structural biology, 212(1), 107581. https://doi.org/10.1016/j.jsb.2020.107581
Risser, F., Collin, S., Dos Santos-Morais, R., Gruez, A., Chagot, B., & Weissman, K. J. (2020). Towards improved understanding of intersubunit interactions in modular polyketide biosynthesis: Docking in the enacyloxin IIa polyketide synthase. Journal of structural biology, 212(1), 107581. https://doi.org/10.1016/j.jsb.2020.107581
![]() Kammerscheit X., Hecker A., Rouhier N., Chauvat F. & Cassier-Chauvat C. (2020). Methylglyoxal Detoxification Revisited: Role of Glutathione Transferase in Model Cyanobacterium Synechocystis sp. Strain PCC 6803. Molecular Biology and Physiology, 11, e00882-20.
Kammerscheit X., Hecker A., Rouhier N., Chauvat F. & Cassier-Chauvat C. (2020). Methylglyoxal Detoxification Revisited: Role of Glutathione Transferase in Model Cyanobacterium Synechocystis sp. Strain PCC 6803. Molecular Biology and Physiology, 11, e00882-20.
![]() Roret T., Alloing G., Girardet J.M., Perrot T., Dhalleine T., Couturier J., Frendo P., Didierjean C. & Rouhier N. (2020). Sinorhizobium meliloti YrbA binds divalent metal cations using two conserved histidines. Biosci. Reports, 40, BSR20202956
Roret T., Alloing G., Girardet J.M., Perrot T., Dhalleine T., Couturier J., Frendo P., Didierjean C. & Rouhier N. (2020). Sinorhizobium meliloti YrbA binds divalent metal cations using two conserved histidines. Biosci. Reports, 40, BSR20202956
![]() Delannoy M., Girardet J.-M., Djelti F., Yen F.T. & Cakir-Kiefer C. (2020). Affinity of chlordecone and chlordecol for human serum lipoproteins. Environmental Toxicology and Pharmacology, 80, 103486.
Delannoy M., Girardet J.-M., Djelti F., Yen F.T. & Cakir-Kiefer C. (2020). Affinity of chlordecone and chlordecol for human serum lipoproteins. Environmental Toxicology and Pharmacology, 80, 103486.
![]() Vicente C.M., Girardet J.M., Hôtel L. & Aigle B. (2020). Molecular dynamics to elucidate the DNA-binding activity of AlpZ, a member of the gamma-butyrolactone receptor family in S. ambofaciens. Frontiers in Microbiology: Antimicrobials, Resistance and Chemotherapy, 11, 1255.
Vicente C.M., Girardet J.M., Hôtel L. & Aigle B. (2020). Molecular dynamics to elucidate the DNA-binding activity of AlpZ, a member of the gamma-butyrolactone receptor family in S. ambofaciens. Frontiers in Microbiology: Antimicrobials, Resistance and Chemotherapy, 11, 1255.
![]() Perrot T., Schwartz M., Saiag F., Salzet G., Dumarcay S., Favier F., Gerardin P., Girardet J.M., Sormani R., Morel-Rouhier M., Amusant N., Didierjean C. & Gelhaye E. (2018). Fungal glutathione transferases as tools to explore the chemical diversity of Amazonian wood extractives. ACS Sustainable Chemistry & Engineering, 6, 13078-13085
Perrot T., Schwartz M., Saiag F., Salzet G., Dumarcay S., Favier F., Gerardin P., Girardet J.M., Sormani R., Morel-Rouhier M., Amusant N., Didierjean C. & Gelhaye E. (2018). Fungal glutathione transferases as tools to explore the chemical diversity of Amazonian wood extractives. ACS Sustainable Chemistry & Engineering, 6, 13078-13085
Equipment referents
















